Abstract
Sickle cell disease (SCD) is due to a mutation in the β-globin (HBB) gene causing the production of the sickle β S-globin chain. The sickle Hb (HbS, a 2β S2) polymerizes, leading to the formation of sickle-shaped red blood cells that cause vaso-occlusions and organ damage. Transplantation of autologous hematopoietic stem/progenitor cells (HSPCs) transduced with lentiviral vectors (LV) expressing an anti-sickling β-globin transgene (βAS LV) is a promising curative treatment; however, it is partially effective in SCD patients, who still present elevated HbS levels.
Here, we aim to improve LVs to boost therapeutic β-like globin levels without increasing the mutagenic vector load in HSPCs. We developed 2 novel LVs expressing βAS together with an artificial microRNA (amiR) targeting either the fetal Hb (HbF) repressor BCL11A (βAS/amiRBCL11A) or the β S-globin (βAS/amiRHBB). By downregulating BCL11A, amiRBCL11A re-activates the expression of the endogenous anti-sickling fetal γ-globin, which, together with βAS, should improve the clinical course of SCD; β S-globin downregulation should favor βAS incorporation in Hb tetramers, increase therapeutic Hb levels and ameliorate the SCD phenotype.
First, we developed βAS/amiRBCL11A LV by inserting the amiR in multiple position of the βAS intron 2 under the control of HBB promoter/enhancers to limit BCL11A downregulation to the erythroid lineage and reduce potential amiR-related cellular toxicity and off-target effects. We showed that amiR insertion site did not affect LV titer nor βAS expression in a human erythroid cell line (HUDEP2). BCL11A downregulation in HUDEP2 led to γ-globin gene de-repression and a high proportion of HbF + cells (RTqPCR, HPLC, flow cytometry). Importantly, the total amount of therapeutic β-like globins was substantially higher in βAS/amiRBCL11A LV- than in βAS LV-transduced cells, with no impairment in cell viability or erythroid differentiation.
In parallel, we designed 17 amiRs targeting HBB and generated the corresponding βAS/amiRHBB LVs. We tested these LVs in HUDEP2 and selected 2 amiRs efficiently downregulating β-globin at mRNA and protein levels (RT-qPCR and Western Blot). Of note, we modified the βAS transgene by inserting silent mutations that prevent its recognition by the amiR (βASm).
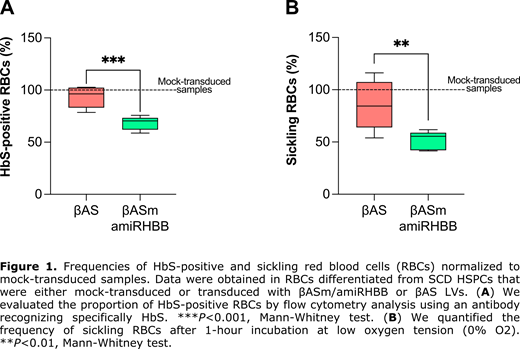
Finally, we tested βAS/amiRBCL11A and βAS/amiRHBB LVs in HSPCs from SCD patients. HSPC-derived erythroid cells transduced with βAS/amiRBCL11A LV showed increased HbF levels, although HbS levels remained high. To further reduce β S-globin levels, we targeted the β S-globin mRNA using the βAS/amiRHBB LV. Efficient HSPC transduction by βASm/amiRHBB LV led to a substantial decrease of β S-globin transcripts in HSPC-derived erythroid cells compared to the βAS LV-transduced cells (RTqPCR) at a VCN/cell of 2. Notably, the amiR specifically down-regulated β S-globin, without affecting βAS expression. In βASm/amiRHBB- vs βAS LV-transduced cells, HPLC analysis showed that β S-globin downregulation led to a significant decrease of HbS, which represented 58% and 71% of the total Hb, respectively). This was associated with a significant increase of the therapeutic Hb in βASm/amiRHBB LV- vs βAS LV-transduced erythroid cells (38% and 27% of the total Hb, respectively). Importantly, we observed a substantial reduction of the proportion of HbS-positive cells in βASm/amiRHBB- vs βAS LV-transduced samples (from 96% to 70%; Figure 1A). The increased incorporation of βAS in Hb tetramers and the decrease in β S-globin led to a better correction of the sickling phenotype in mature RBCs derived from HSPCs transduced with βASm/amiRHBB LV- compared to βAS LV (55% and 84% of sickling cells, respectively; Figure 1B). A clonal assay of hematopoietic progenitors showed no impairment in HSPC viability and differentiation towards the erythroid and myeloid lineages upon transduction with bifunctional LVs. βASm/amiRHBB LV showed a standard lentiviral integration profile. Finally, we performed RNAseq to further evaluate the safety of our therapeutic strategy.
In conclusion, we created a LV able to concomitantly silence the β S-globin and express βAS, achieving clinically relevant levels of therapeutic Hb and efficient correction of the sickling phenotype. Therefore, the combination of gene addition and gene silencing strategies can improve the efficacy of current therapeutic approaches, representing a novel treatment for SCD.
Cavazzana: Smart Immune: Other: co-founder.